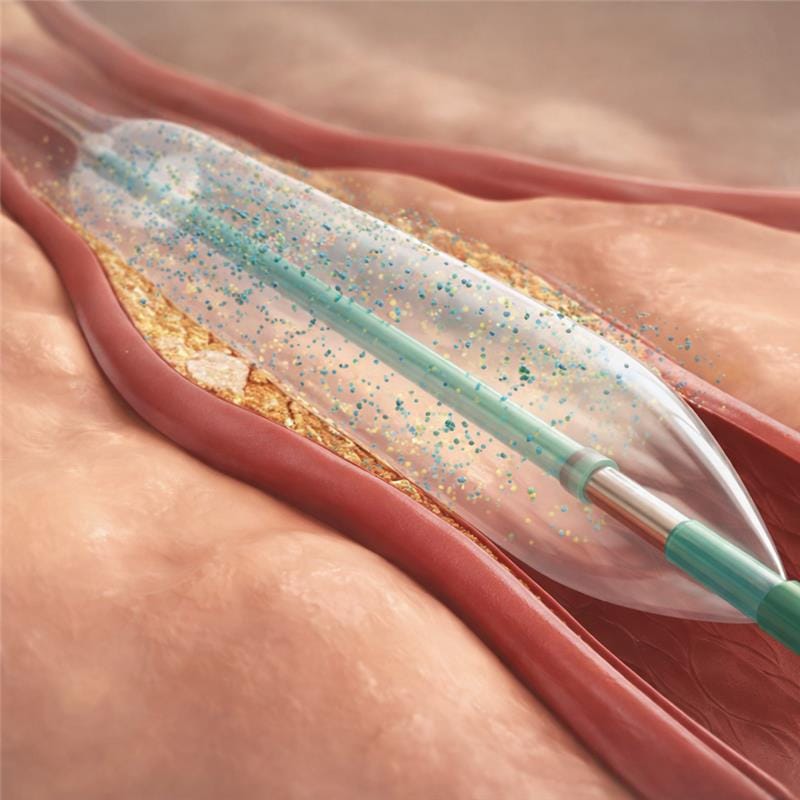
Drug-coated balloons (DCBs) have been designed to treat vascular narrowing while addressing the biological cause of restenosis without leaving a permanent implant behind. During balloon inflation, an antiproliferative drug, most commonly paclitaxel, is delivered directly to the vessel wall. Owing to its high lipophilicity, paclitaxel is rapidly absorbed and retained within the arterial tissue after short exposure, where it inhibits smooth muscle cell proliferation and neointimal hyperplasia, the key mechanisms responsible for vessel re-narrowing following angioplasty.
Unlike drug-eluting stents (DES), which rely on a permanent metallic scaffold to provide mechanical support and sustained drug release, DCBs achieve their therapeutic effect through transient local drug delivery while allowing the vessel to heal naturally. The absence of a permanent implant reduces chronic inflammatory stimulus, preserves native vessel anatomy and vasomotion, and avoids the cumulative metal burden that may complicate future interventions. This “leave nothing behind” approach is particularly advantageous in anatomies where stent placement is less desirable or where repeated interventions are anticipated.
Unlike stent-based therapies, DCBs provide:
• Homogeneous drug delivery to the treated vessel segment
• No permanent metallic scaffold, reducing chronic inflammation
• Preservation of native vessel anatomy and vasomotion
• Reduced stimulus for late neointimal growth
DCBs are supported by robust clinical evidence across multiple vascular studies. In the coronary circulation, they are well established for the treatment of in-stent restenosis and are increasingly used in small-vessel disease, where stent implantation is associated with higher restenosis rates. In the BASKET-SMALL 2 trial, treatment with a drug-coated balloon demonstrated non-inferior outcomes compared to drug-eluting stents, with major adverse cardiac event rates of 7.5 percent for DCBs and 7.3 percent for DES at 12 months, and identical rates of 15 percent at three years. Angiographic data further support the biological efficacy of DCB therapy. In the PICCOLETO II trial, late lumen loss at nine months was significantly lower with DCB treatment at 0.04 millimetres compared to 0.17 millimetres with DES, indicating reduced neointimal growth.
In the setting of coronary in-stent restenosis, multiple randomised studies and meta-analyses have shown that DCBs achieve clinical outcomes comparable to repeat DES implantation while avoiding the placement of additional metal layers. This is a critical consideration in patients with recurrent restenosis, where excessive stent layering can impair vessel compliance and long-term outcomes. Beyond the coronary arteries, drug-coated balloons have demonstrated clear benefits in peripheral arterial disease, particularly in the femoropopliteal segment and below-the-knee vessels, where long stents are exposed to mechanical stress and higher fracture risk. In these territories, DCBs consistently reduce restenosis and repeat revascularisation rates compared to plain balloon angioplasty, while maintaining vessel patency without the drawbacks associated with permanent implants.
Collectively, these data prove how drug-coated balloons function as a biological barrier to restenosis by suppressing pathological vascular healing while enabling physiological recovery of the vessel. By combining mechanical dilatation with targeted drug delivery and eliminating the need for permanent scaffolds, DCB technology represents a scientifically grounded and clinically validated approach to modern endovascular therapy.
At AVA DCB, the focus remains on advancing science-driven drug-coated balloon solutions that integrate pharmacology, coating technology, and vascular biology to support durable outcomes without unnecessary implant burden.
Want to speak to one of our experts? Contact us by dropping us an email on info@avadcb.com or ssibartie@avadcb.com.
“This post is intended for scientific education and does not constitute clinical guidance.”
References:
Scheller B. et al. Paclitaxel balloon coating for prevention and therapy of restenosis, Circulation, 2004
Jeger et al., Drug-Coated Balloons for Coronary Artery Disease, 2020
Scheller et al., Long-Term Follow-Up After Treatment of Coronary In-Stent Restenosis with a Paclitaxel-Coated Balloon Catheter, 2012
Giacoppo.D et al., Coronary Drug-Coated Balloons for De Novo and In-Stent Restenosis Indications, 2023
Scheller B. Et al., Treatment of Coronary In-Stent Restenosis with a Paclitaxel-Coated Balloon Catheter, 2006
Byrne R et al., Drug-coated balloon therapy in coronary and peripheral artery disease, Nature Reviews Cardiology, 2014
Rittger H, et al., Long-term outcomes after treatment with a paclitaxel-coated balloon versus drug-eluting stent, Journal of the American College of Cardiology, 2012
Alfonso F, et al., Drug-coated balloon treatment of in-stent restenosis: A clinical update, Journal of the American College of Cardiology, 2014
Ali R, et al., Drug-coated balloons for coronary artery disease: A systematic review and meta-analysis. European Heart Journal, 2019
Written by Sheena Sibartie, Senior Lab Engineer